Home - About - Research Data Management - Guidelines
Welcome to our “Guidelines” page! Here, you will find the principles and best practices that govern our research data management activities. These guidelines are designed to ensure the consistency, efficiency and, quality of projects at the Lady Davis Institute for Medical Research. Whether you are a collaborator, partner or affiliated researcher, this section will help you better understand our approach and align yourself with our policies and standards. Our policies and standards provide guidelines, characteristics or requirements for processes or services.
Please take the time to review them to ensure a smooth and seamless experience throughout the lifecycle of your research projects.
Research Data Management (RDM) refers to the processes involved in handling data generated during research activities. It covers everything from how data is planned, created, organized, stored, and preserved to how it is shared. Effective RDM ensures that data is accurate, reliable, secure, and accessible, both during the research project and after its completion. The Research Data Lifecycle (RDL) is a framework that describes the different stages the data undergoes in a research project.
The Research Data Lifecycle and Research Data Management (RDM) are tightly interwoven. RDM provides the framework for managing data throughout each stage and encompasses the policies, processes, and tools that ensure data is handled responsibly and effectively at each point.
These two concepts offer a comprehensive approach to ensuring data governance in the research field toward contributing to the broader goals of transparency, reproducibility, and collaborative science.
This document presents the policies, processes, guidelines and tools that the Lady Davis Institute (LDI) and the Jewish General Hospital – CIUSSS West-Central Montreal (CCOMTL) have implemented to facilitate research data management. The sections below explain the organization of services, procedures, and standards for managing research data in compliance with the legal framework and ethical guidelines. This reference document for research data management summarizes the services and infrastructures at the LDI, which provide researchers with the tools to manage their research data responsibly throughout the project lifecycle.
Please see Appendix A for a full list of concepts definitions.
The diagram below shows an overview of the different phases of a research project’s life cycle and the procedures or standards required for each stage.
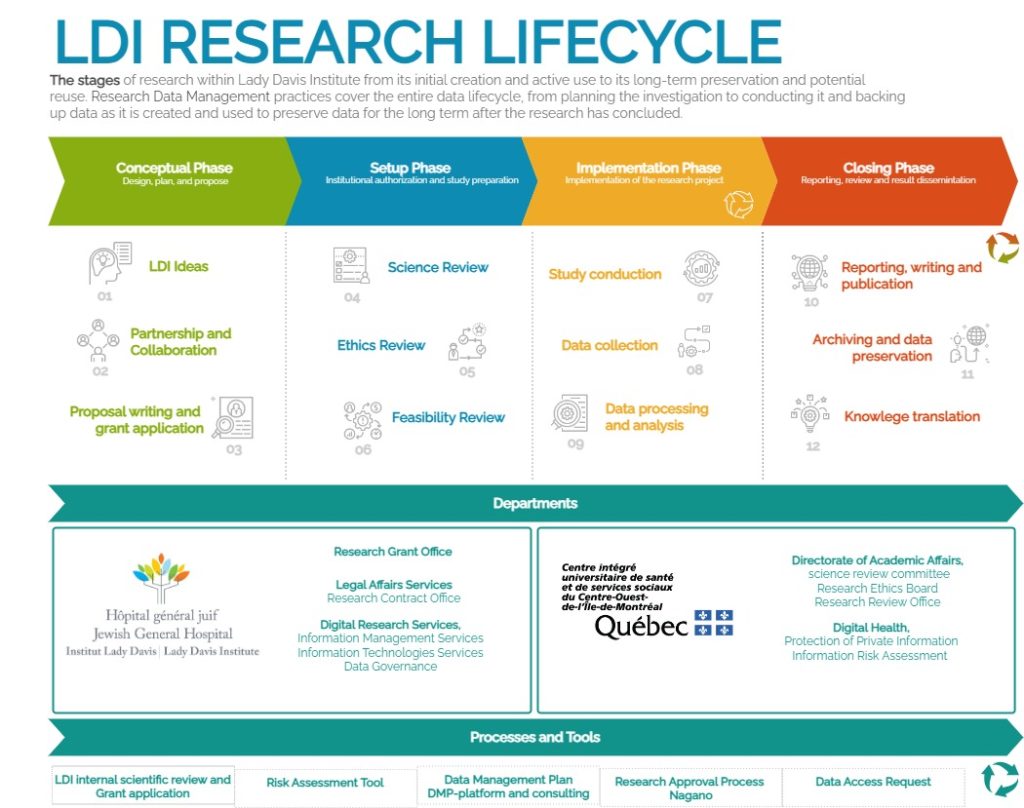
During the Conceptual phase, the researcher designs, plans, and defines the research proposal to obtain funding. The conceptual phase of the research lifecycle includes three main steps. The procedures, services, and tools currently provided by the Lady Davis Institute are summarized below.
At this stage, researchers identify their research question and consider how to structure their research project. It is the step to estimate the needs and plan the research project. Several departments at the Lady Davis Institute assist researchers during this stage.
The Research Grants Office offers assistance such as :
Pre-proposal reviews
The Information Technologies Services and Information Management Services provide assistance for:
At this stage, the Principal Investigator (PI) must build the collaboration or partnership framework to conduct research. Researchers plan the research project and identify whether they must establish partnership and collaboration agreements or contracts with the various research stakeholders. In addition, researchers must comply with the legal provisions relating to the specific nature of their research project (laws, funding agency requirements, institutional regulations, policies, and procedures). Here are the support services available within LDI.
The Legal Affairs Services (Research Contracts Office) offers assistance to :
Draft, review, and negotiate various agreements concerning research projects
Provide legal advice to ensure research agreements comply with applicable laws, regulations, guidelines, governmental directives, funding agency requirements, and institutional regulations, policies, and procedures.
The final stage of the conceptual phase involves writing a proposal and applying for a Grant. At LDI, researchers must undergo the scientific internal peer review process before submitting the grant application. Ideally, researchers should define a data management plan during this phase to plan their research project and the data lifecycle. The Research Grants Office and Information Management Services (Digital Research Services) offer support services.
The Research Grants Office offers assistance for :
Identifying available funding opportunities
Developing research grants
Preparing the scientific Internal peer-review
Institutional letter of support and signature for a grant application
Providing support during the post-awards process
The Information Management Services (Digital Research Services) offer assistance throughout a request ticketing system for various such as :
Guidance on the project approval process within the Jewish General Hospital (JGH) / CIUSSS West-Central Montreal Institutional Approval Process
Advice on IT policy compliance and IT-related expenses costing
The Research Review Office (RRO) provides:
Support for preparing a research project for evaluation
Support to coordinate the three levels of approval before the research project’s deployment within the institution is authorized by the CIUSSS: scientific approval, ethics approval and approval of feasibility
Nagano is the platform used to submit the research project and coordinate assessment processes.
Science review committee – CCOMTL
Evaluate research protocols that have not already obtained a scientific review from granting agencies recognized by the MSSS.
Research Ethics Board – CCOMTL
Draft, review, and negotiate various agreements concerning research projects
Provide legal advice
Institutional Feasibility – CCOMTL
Evaluate available material and human resources on-site research projects
Perform Data Security Review, if needed
Privacy Impact Assessment (EFVP – Evaluation des facteurs de vie Privée)
For further questions or information about the Research Ethics review or for the Nagano platform, please contact cer@jgh.mcgill.ca
For further questions or information about the Feasibility review process, please contact convenance@jgh.mcgill.ca
Digital Research – Information Management Service offer assistance such as :
Provide a toolset for creating and updating a research project’s data management plan (DMP). The DMP platform is under development and will be available for researchers in 2025.
This phase represents the execution of the research project. It involves implementing the research protocol and the data management plan developed during the planning phase. It is the phase where the research team gathers the data needed to answer the research questions. This involves data collection, experiments, or clinical trials following an established research protocol. Researchers must document and manage the data, ensuring accuracy and adherence to quality control standards. Researchers also set up infrastructures, such as data collection tools, software, equipment, and team roles. Throughout this phase, the research team monitors progress, handles any adjustments or unforeseen challenges, and ensures that all activities align with the research protocol and the project’s data management plan.
The activities in the Study Conduction phase vary based on the type of study (e.g., experimental, observational, clinical trial, etc.) but typically start with the recruitment of participants. When the research subjects are human, this process involves obtaining informed consent from participants. Within the CIUSSS du Centre-Ouest-de-l’Île-de-Montréal (CCOMTL), if the project requires access to patient data without individual patient consent, the PI affiliated with Lady Davis Institute must submit a Data Access Request (DAR) using the LDI DAR platform. For Further information about the DAR process, please contact us throughout the LDI Ticketing System via the link https://itservicedesk.ladydavis.ca/a/catalog/request-items/87.
Lady Davis Institute provides some Digital Research Services in collaboration with CCOMTL’s Digital Health Department :
Data Access Request Platform. This toolset is under development and will be available for researchers in 2025
Clinical Data Repository is currently accessible for LDI-affiliated researchers pending a request. Please submit your Data request here.
Data collection can involve surveys, experiments, clinical measurements, interviews, or dataset provision for projects using clinical or patient records. This includes data entry, organization, validation, and backup, as well as protecting sensitive or confidential information following privacy and ethical standards. Maintain data integrity, confidentiality, and adherence to ethical guidelines. At this stage, It’s essential to ensure data quality. This phase may also involve managing any issues that arise during the study, such as unforeseen challenges.
In case the research project uses clinical records, the Data provision phase involves managing how the data will be accessed and shared, particularly with external researchers or institutions. This phase focuses on the legal, ethical, and logistical considerations for sharing or distributing the study’s data.
Lady Davis Institute provides some Digital Research Services for LDI-affiliated, such as :
Electronic Data Capture with RedCap or other secured data collection tools. For further details, please contact
Clinical Data Repository for further details, please submit a data request at https://itservicedesk.ladydavis.ca/a/catalog/request-items/87
It is also possible for the researcher to request access to the medical archive of the Jewish General Hospital (JGH). For further details, please contacthttps://www.ciussswestcentral.ca/programs-and-services/ciusss-medical-records/.
Ethical Considerations and Permissions: Before data can be accessed by external parties or the research team, the PI must ensure that data sharing complies with ethical guidelines. This may involve:
Ensuring that participant privacy and confidentiality are protected.
Securing any required permissions from participants or institutions.
Adhering to any legal or institutional restrictions related to data access.
Data Sharing Agreements (DSAs): If access is granted, formal agreements outline the terms and conditions of data sharing.
If you need assistance with any data-sharing agreement, please contact the LDI’s legal department at research.legal.ccomtl@sss.gouv.qc.ca or submit your request via the Research Contract Management System (password required).
These phases involve systematically handling the collected data, preparing it for analysis, and performing statistical or computational analyses to derive insights.
Data processing refers to the steps taken to clean, organize, and transform raw data into a usable format for analysis. In research, raw data often needs to be processed before meaningful insights can be drawn. This phase ensures the data is accurate, consistent, and ready for further investigation.
Once the data is processed, the Data Analysis phase begins. This is where the data is analyzed, allowing researchers to test hypotheses, derive patterns, and extract insights. The analysis phase varies depending on the type of research (qualitative vs. quantitative) and the specific research questions.
Here are services available within LDI for assistance at the stage of Data Processing and Analysis :
The Digital Research – Information Management Services (IMS)
Provides statistical analysis services and Bioinformatics Analysis Services
Get Provision of expertise or strategic advice about software or Software Installation
support for data processing and analysis using Power BI
Please fill out this IMS New Features to Existing Project Request form
All the stages of the implementation phase are iterative. During data processing, issues with the data might be discovered that require going back to earlier steps. Similarly, new questions or issues may arise during data analysis that require further data cleaning or transformation. It is essential to ensure the implementation of the DMP and its update during this phase.
During the closing phase, the findings are disseminated, the data is preserved for future use, and the research’s broader impact is communicated. This phase includes three key steps: Reporting, Writing, and Publication; Archiving and Data Preservation; and Knowledge Translation.
This step involves compiling the research results in a clear and structured manner. The writing should adhere to specific guidelines or publication standards depending on the target journal or audience. Publication ensures that the research is disseminated to the broader scientific community, contributing to the body of knowledge.
Archiving involves storing the raw data, methodologies, and other research materials in a secure and accessible way. Archiving ensures that the research process can be reproduced, validated, or revisited by others in the future. Moreover, the long-term storage and preservation of research data are essential to ensure that it remains accessible and usable in the future.
At this stage, many funding agencies recommend depositing research project data in publicly accessible repositories or archives. Adequate archiving and data preservation ensures a standardized and well-documented format, which enables transparency and reproducibility.
Clarifying the archiving and preservation strategy within the data management plan (DMP) is essential.
Knowledge Translation (KT) refers to the process of making research findings accessible and usable to a broader audience beyond just academics. The goal of KT is to bridge the gap between research and real-world application. This may include policymakers, practitioners, industry professionals, or the general public.
In summary, the closing phase of a research project is focused on making the research widely accessible, ensuring its long-term integrity, and facilitating its practical use.
The Information Management Service (IMS) provides statistical analysis services for compiling abstracts, presentations, and articles published in peer-reviewed journals and for reviewing the statistical analysis section and statistical power of sample size calculations.
Researchers using LDI information technologies assets could benefit from backup and recovery services by submitting their request here.
Please visit McGill University Records Retention Schedule (MURRS) for documentation and archiving guidelines.
LDI does not currently offer a data deposit service. To deposit your research project data, please refer to McGill Dataverse collection with Borealis or the Federated Research Data Repository, a national research data repository managed by the Digital Research Alliance of Canada and freely available to Canadian PIs.
Cybersecurity is the practice of protecting systems, networks, and programs from digital attacks.
The protection of digital information and the integrity of the infrastructure housing and transmitting digital information. More specifically, cyber security includes the body of technologies, processes, practices and response and mitigation measures designed to protect networks, computers, programs and data from attack, damage or unauthorized access to ensure confidentiality, integrity and availability.
Cybersecurity has become a critical concern for individuals, organizations, and governments. The risk of cyber threats grows as we rely more on digital technologies to store, share, and process sensitive information. From protecting personal data to defending against large-scale cyberattacks, cybersecurity is essential to safeguarding digital assets’ integrity, confidentiality, and availability, especially research data.
Effective cybersecurity is not just about installing security software; it’s about adopting a proactive approach to managing risks and building a culture of awareness. Here are some advice :

Find more Cyber Security advice from CIUSSS West-Central Montreal here.
To learn more about information security and cybersecurity best practices, click here.
Find out how knowledgeable you are about cybersecurity by answering a few questions during the Cyber Safe Checkup.
Effective record retention ensures that your research project complies with legal requirements, protects sensitive information, and maintains operational efficiency. Here are some key best practices to follow when managing and retaining records:
Make sure you’re aware of the specific requirements for your field (e.g., healthcare, Canada Health, research) and ensure compliance with data privacy laws such as the Act Respecting Health and Social Services information, Act Respecting the Protection of Personal Information in the private sector, or other relevant regulations.
Organize records by category (e.g., administrative documents, contracts, research data) and classify them based on their importance, sensitivity, and retention requirements.
Create a clear, written record retention policy that outlines how long to keep each type of document, who is responsible for managing records, and how they should be disposed of once they are no longer needed. Reviewing retention policy periodically to ensure it aligns with any legal updates or operational changes is essential.
Store records in a secure, accessible manner. Ensure physical records are stored in fireproof cabinets or safe locations. For digital records, use encrypted storage solutions backed up regularly to prevent loss due to data breaches or technical failures.
 Funding bodies and publishers increasingly require data deposit (i.e. publishing) of research results alongside publication. Making data accessible in a trusted data repository has the following advantages: it allows others to verify your research; encourages others to cite your research; can lead to new contacts from potential collaborators, funders, and other interested parties; provides a securely stored, authoritative copy of your data; and may be found by another research who can then use it in other research.
Funding bodies and publishers increasingly require data deposit (i.e. publishing) of research results alongside publication. Making data accessible in a trusted data repository has the following advantages: it allows others to verify your research; encourages others to cite your research; can lead to new contacts from potential collaborators, funders, and other interested parties; provides a securely stored, authoritative copy of your data; and may be found by another research who can then use it in other research. 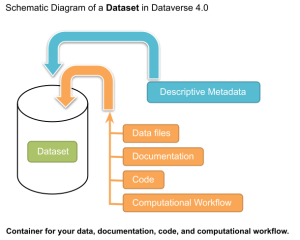
A data repository is a broad term referring to any centralized location where data is stored, managed, and made accessible, designed to hold data types, such as Data archives (e.g., for long-term storage of research data).
A Dataverse is a specific term often referring to a data repository system primarily designed to manage and share research data. It is a specialized platform used for:
Data are facts, measurements, recordings, records, or observations collected by researchers and others, with a minimum of contextual interpretation. Data may be in any format or medium taking the form of text, numbers, symbols, images, films, video, sound recordings, pictorial reproductions, drawings, designs or other graphical representations, procedural manuals, forms, diagrams, workflows, equipment descriptions, data files, data processing algorithms, software, programming languages, code, or statistical records. (Adapted from: Tri-Agency RDM Policy FAQ)
Research data can be any information collected, observed, or created during research activities. It can be in many forms: numerical datasets, text files, lab notebooks, surveys, images, videos, or software code, publication, communications, and administrative files.
As the Lady Davis Institute is a medical research institute, Institutional Data can be defined as two categories of data generated during research activities. There are :
Scientific and Clinical Data: This category encompasses all data generated from research project activities. These data are crucial for supporting scientific inquiry and validating research findings. The Scientific and Clinical institutional data include all research outputs, survey responses, interview records, clinical trial results, patient records, imaging data, and laboratory data generated by medical research activities.
Operational and Collaborative Data: This category includes data related to the operational and administrative functions and data from external collaborations. This covers administrative, financial, legal, and regulatory data and information from partners or external collaborations. The operational and collaborative data ensures the traceability of the research projects’ operations at the Lady Davis Institute.
Data Lifecycle refers to all the stages in the existence of data from creation to destruction. The data lifecycle provides a high-level overview of the stages involved in successful management and preservation of data for use and reuse. This broadly includes the following stages: Plan, Create, Process, Analyze, Disseminate, Preserve and Reuse. (Adapted from: CASRAI Definition of Data Lifecycle, DataOne, & Alliance-Portage)
The data lifecycle is a high-level overview of the stages for successfully managing and preserving data for use, reproducibility and transparency. A data lifecycle consists of a series of stages that start with planning, creating, storing, processing, sharing, and archiving and end with the preservation or destruction of data. A data lifecycle approach ensures identifying and planning the necessary data management stages (Higgins, 2008).
The research data lifecycle (RDL) is the roadmap for managing data from the start of a research project to its long-term reuse. The research data lifecycle consists of seven distinct stages. The term “RDL” encompasses all phases of data existence, ranging from its initial creation to its eventual destruction. This broadly includes the following stages: Planning, Creation, Processing, Analysis, Sharing or Dissemination, Preservation and Reuse. (Adapted from CASRAI Definition of Data Lifecycle, DataOne, & Alliance-Portage).
Data Management encompasses all the activities involved in collecting, storing, processing, analyzing, sharing and using data within an organization. These processes must be secure, efficient and compliant with laws, policies, and other relevant regulations.
Research Data Management refers to the processes applied through the lifecycle of a research project to guide the collection, documentation, storage, sharing and preservation of research data. (Adapted from: Tri-Agecy RDM Policy FAQ and Alliance-Portage Definition)
Hence, Research Data Management provides the necessary guidance, infrastructure, and practices to manage the data at each stage of a research project and data lifecycle.
Researcher means a person to whom the CCOMTL has awarded research privileges, excluding research personnel or students. (From: Regulatory framework for research involving humans at the Centre intégré universitaire en santé et services sociaux du Centre-Ouest-de-l’Île-de-Montréal)
You have the power to make a difference! Your gift will support vital research at the Lady Davis Institute for Medical Research (LDI) that will translate into disease prevention, improved diagnoses, earlier detection, new and enhanced therapies, a better quality of life, wellness and hope for all of us.
Faire la différence, vous avez ce don! Votre contribution soutiendra la recherche essentielle menée à l’Institut Lady Davis qui permettra la prévention des maladies, des diagnostics plus précis, des dépistages plus rapides, des traitements innovants et plus performants, une meilleure qualité de vie, le bien-être et l’espoir pour nous tous.
Copyright © 2024 | Lady Davis Institute for Medical Research/Jewish General Hospital
Conception et développement : Yankee Media